|
NMR (nuclear magnetic resonance) spectroscopy is used extensively in organic chemistry to aid in the determination of molecular structure. NMR is a form of absorption spectroscopy which uses low frequency radiofrequency waves. Although the structures of some organic molecules may be determined using only its NMR spectrum, most complex molecules must be studied using NMR along with some other forms of spectroscopy and analysis (Wade 544).
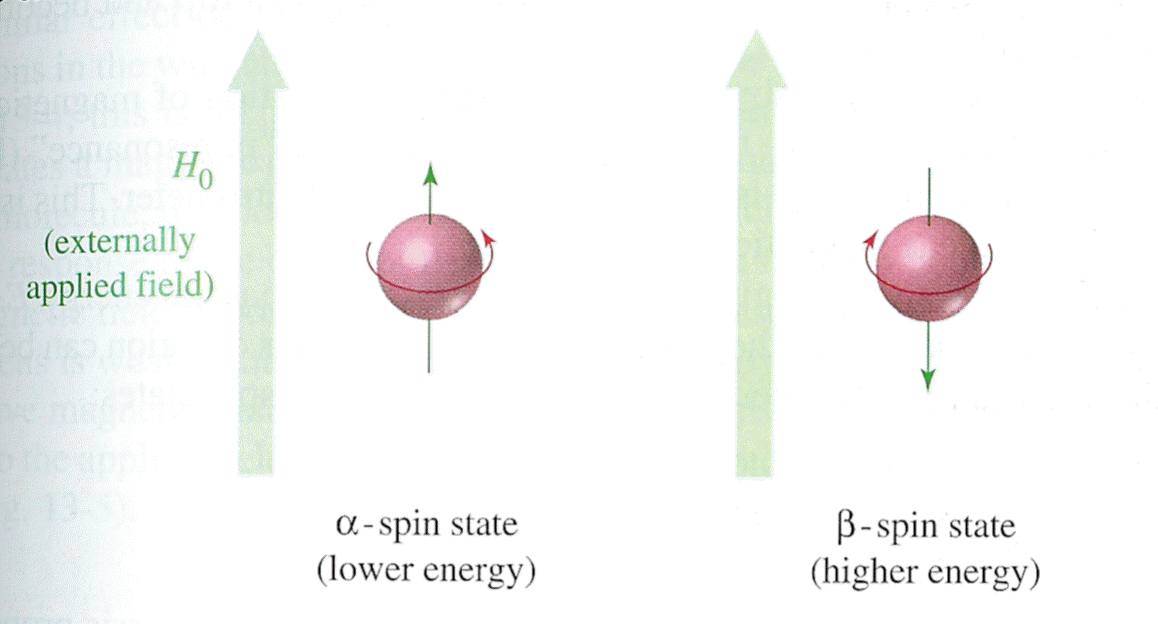
Unless otherwise specified, the magnetic resonance in NMR refers to the magnetic resonance of the protons in the molecule. Since a proton has an odd atomic number of 1, its nuclear spin can be detected by a NMR spectrometer (Wade 544). The terms "proton" and "hydrogen" are often used interchangeably in literature dealing with NMR. The spinning of the hydrogen nucleus produces a magnetic field whose overall direction is termed the magnetic moment. Under normal conditions, magnetic moments have random orientations, but when placed in the presence of an external field (H0), the nuclei align themselves either along or against the direction of H0. Protons whose magnetic moments are aligned with the external field are lower in energy than those whose magnetic moments oppose the external field. The low-energy state is called the alpha-spin state, and the high-energy state is termed the beta-spin state (Wade 545).
|
| Image taken from L.G. Wade Jr. Organic Chemistry, 4th ed. 1999 |
When the resonance frequency of the external magnetic field matches the energy difference between the alpha-spin and beta-spin states, a nucleus may move (flip) from the alpha-state to the beta-state. The amount of energy absorbed in this process is detected by the spectrometer and registers as a peak on the spectrum (Wade 546).
There are several items to note when observing an NMR spectrum:
- Number of signals
- Position of peaks
- Intensity of signals
- Splitting of signals.
The number of signals tells how many different types of hydrogens are present in the molecule. Though they may seem equivalent, a hydrogen connected to a carbonyl carbon is different from a hydrogen in a methyl group. Peak position is indicative of the chemical environment of the proton in question. This is measured in chemical shift (delta), which is the difference between the ratio of the frequency of the observed proton with the reference molecule (usually tetramethylsilane, TMS). The intensity of the signal is dependent upon the number of hydrogens present for that particular peak, and the splitting (number of peaks in a set) represents the environment of the proton with respect to its nearby (adjacent) protons (Gallo, lecture notes).
|